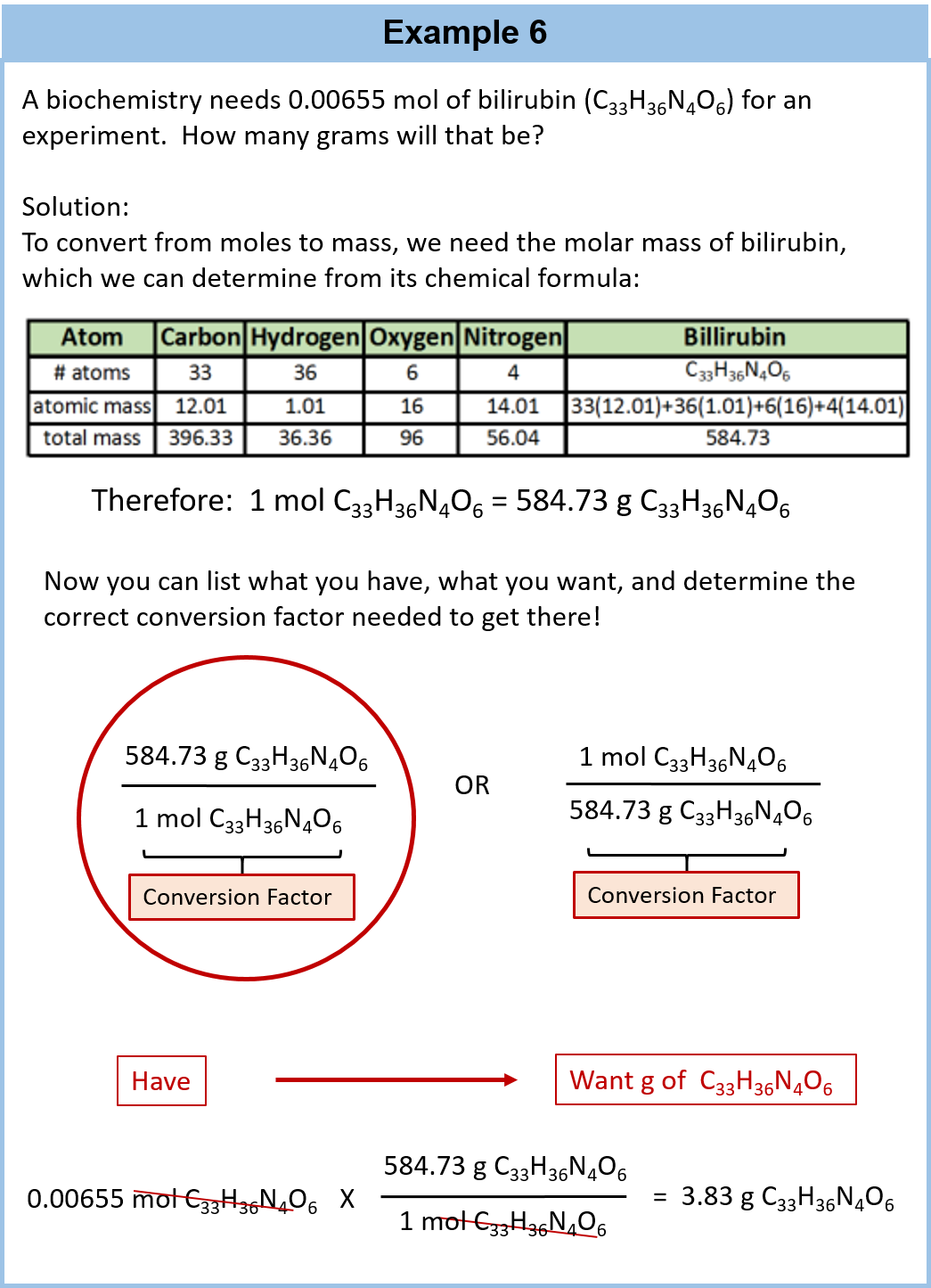
Mass Of One Atom Of Oxygen
veena Vasandani answered this
Please find below the solution to the asked query:
Answer is option d.
One atom of O has 8 protons and 8 neutrons. Mass of proton or neutron is 1.66 10-24 g.
Mass of 16 nucleons = 16 ( 1.66 10-24) g
Mass of an oxygen atom is 16 amu and in terms of gram atomic mass mass of 1 mole is 16 g.
Mass of 1 oxygen atom = 16/ 6.022 X 1023 g {1 mole contains 6.022 X 1023 particles)
Hope this information will clear your doubts about mass of an oxygen atom.
If you have any more doubts just ask here on the forum and our experts will try to help you out as soon as possible.
Regards
Reyan Dutta answered this
Mass Of One Atom Of Oxygen Is Mcq
From www.PhysicsAccordingtoPalladino.orgCalculating the Mass of an Oxygen Atom and molecule from the periodic table. Mass of one atom of oxygen is (a) 23 16 g 6.023 10 × (b) 23 32 g 6.023 10 × (c) 23 1 g 6.023 10 × (d) 8u ← Prev Question Next Question → 0 votes 862 views.
The atomic mass unit is the system of measurement designed to identify each individual unit of mass in atoms and molecules. Also known as a dalton, the atomic mass unit is a universally-applied measurement based on 1/12 the total mass of a single carbon-12 atom. This means that a carbon-12 atom has the atomic mass of 12 daltons. The designation for a standard atomic mass unit is u or Da. Atomic mass units are used as the system of measurement in every science, except for those involving biology and biochemistry, which use the dalton designation.
One convenient aspect of atomic mass units is that, while based on carbon mass, a single unit is also equal to one hydrogen atom. This is because the combined mass of a single proton and neutron, the composition of a hydrogen atom, is equal to the measurement. Electrons, being only 1/1836 the mass of a proton, are essentially negligible to the overall mass of an atom.

Mass Of One Atom Of Oxygen
One of the most problematic aspects to using the atomic unit of mass to define atoms is that it does not account for the energy that binds together an atom's nucleus. Unfortunately, this is not a fixed mass due to the differences between each different types of atom. As more protons, neutrons and electrons are added to an atom to create a new element, the mass of this binding energy changes. This means that the measurement can be said to be a rough approximation rather than an exact constant.
Apc by schneider electric mobile phones & portable devices driver download. One of the main uses for the atomic mass unit involves its relationship with moles. A mole is the complete physical quantity of a single unit of a substance. For example, a single water molecule, comprised of two hydrogen atoms and a single oxygen atom, is a mole of water. This means that it has the atomic mass of all three atoms.
Mass Of One Atom Of Oxygen Age
The establishment of the atomic mass unit was first started by a chemist name John Dalton in the early 1800s. He used a single hydrogen atom as the platform for the measurement. However, this was altered by Francis Aston with his invention of the mass spectrometer in the late 1800s. Aston defined an atomic mass unit as being 1/16 the mass of a single oxygen-16 atom. It wasn't until 1961 that the International Union of Pure and Applied Chemistry defined the modern applications of the measurement and linked it to carbon-12.
