29-04-2021
If An Atom Gains Electrons It Becomes A
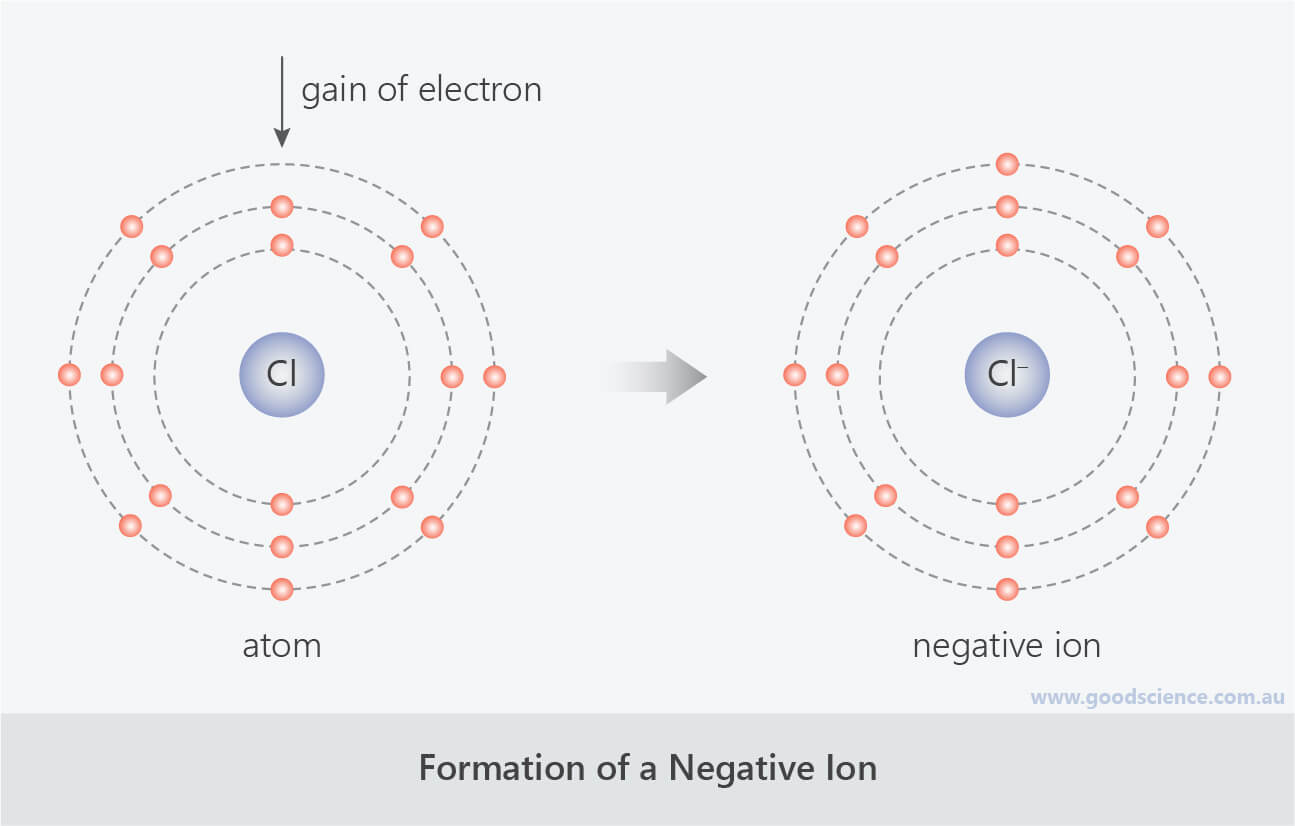
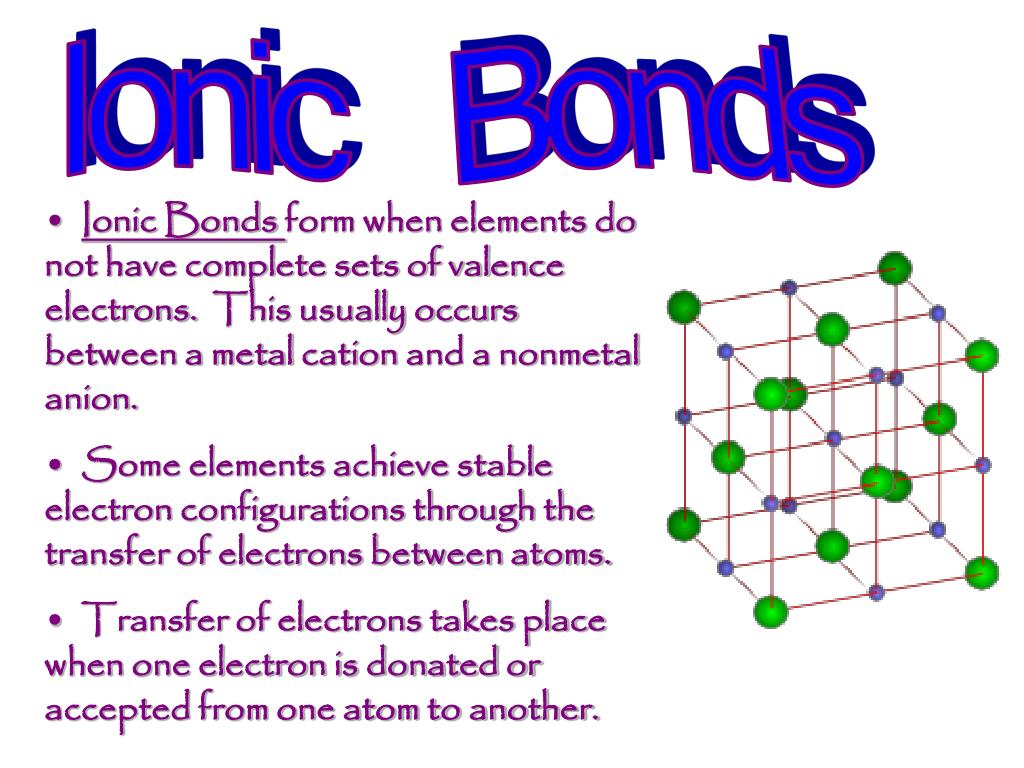
Get an answer for 'Atoms of which types of elements tend to gain electrons? Atoms of which types of elements tend to lose electrons?' And find homework help for other Science questions at eNotes. Which of the following describes what happens when an atom becomes an ion with a 2- charge? A) the atom gains 2 protons b) the atom loses 2 protons c) the atom gains 2 electrons d) the atom loses 2 electrons. However, if something happens to make an atom lose or gain an electron then the atom will no longer be neutral. An atom that gains or loses an electron becomes an ion. If it gains a negative electron, it becomes a negative ion. If it loses an electron it becomes a positive ion (see page 10 for more on ions).
- When An Atom Gains An Electron It Becomes A Positive Ion True Or False
- If An Atom Gains Electrons It Becomes A Gas
- When An Atom Loses Or Gains Electrons It Becomes A
- If An Atom Gains Electrons It Becomes A Part
| Atomic Timeline 1895-1960 | | | It's a Question of Physics | | | What is My Annual Radiation Dose? | | | About The Exhibit |
(click for larger image) The Rutherford Atom for Carbon Ernest Rutherford's original atomic model is now understood to be inaccurate, but it retains its meaning as an icon today. The nucleus consisting of protons and neutrons, here shown in red, is surrounded by orbiting electrons, shown in blue. (click for larger image) The Schrodinger Atom for Carbon Erwin Schrodinger's model is a more correct representation. It includes electron clouds. | An atom is the smallest particle into which an element can be divided without losing its chemical identity. Atoms consist of a heavy central nucleus surrounded by a cloud of negatively charged particles called electrons. The nucleus contains positive particles (protons) and electrically neutral particles (neutrons). The number of protons is called the atomic number. This number uniquely identifies each chemical element. In turn, protons and neutrons are composed of quarks. An element is a chemical substance that is made up of single kind of atom. Iron, carbon, and hydrogen are all elements. A molecule is formed when two or more atoms of any kind of element are joined together chemically. If a molecule contains two or more different elements, it is known as a compound. A water molecule is a compound of the elements hydrogen and oxygen. If an atom or molecule becomes electrically charged by gaining or losing one or more electrons, it becomes an ion. If the atom gains electrons, it has a negative charge. If it loses electrons, it has a positive charge. | (click for larger image) Bohr Model of Calcium Bohr originally developed his atomic model to demonstrate the way in which electrons of hydrogen atoms changed orbits. Physicists today know that this version is not completely accurate, but it is still used because it offers a clear explanation of atomic structure at an introductory level. (click for larger image) Bohr Model of a Calcium Ion The calcium molecule above has gained two negatively charged electrons to become a calcium ion. |
 « Back to Questions
« Back to QuestionsLinda Hall Library
When An Atom Gains An Electron It Becomes A Positive Ion True Or False
5109 Cherry StreetIf An Atom Gains Electrons It Becomes A Gas
When An Atom Loses Or Gains Electrons It Becomes A

If An Atom Gains Electrons It Becomes A Part
Kansas City, MO 64110