Atomic Number Of Scandium
- Atomic Number For Scandium To Zinc
- Sc Element
- How Many Electrons Does Scandium Have
- Melting Point Of Scandium
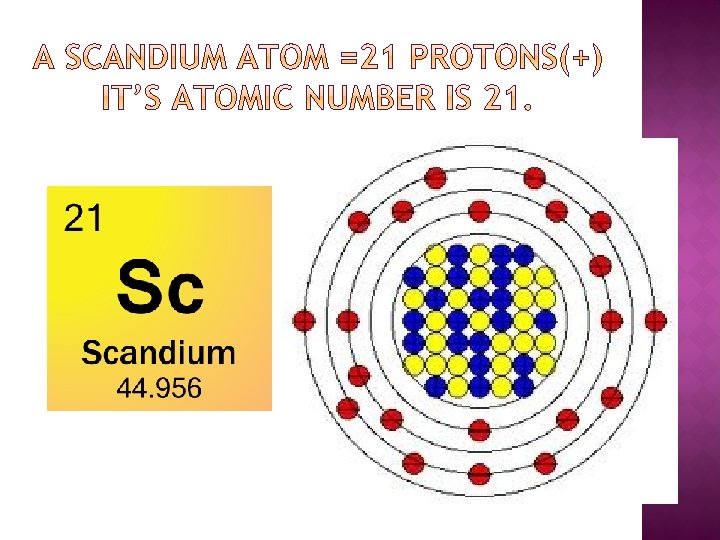
Scandium atoms have 21 electrons and the shell structure is 2.8.9.2. The ground state electron configuration of ground state gaseous neutral scandium is Ar. 4s 2 and the term symbol is 2 D 3/2.
Atomic Number of Scandium is 21.
- You, Sir, could have impregnated google with the question and an answer would have been delivered to you within a second. However, you chose to go into a ‘question and answer’ website and whisper this feeble little question amidst the enormous hue.
- Name: Scandium: Symbol: Sc: Atomic Number: 21: Atomic Mass: 44.95591 atomic mass units: Number of Protons: 21: Number of Neutrons: 24: Number of Electrons: 21.
- Scandium is a chemical element with atomic number 21 which means there are 21 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
- What is the atomic no. Of scandium is 21, it is represented by Sc. Lars Nilson have discovered it in 1879. Atomic Mass os Scandium is 44.95591 amu, it have 21 protons/electrons, Hexagonal is its Crystal Structure & color is silvery. Here is its atomic structure.
Chemical symbol for Scandium is Sc. Number of protons in Scandium is 21. Atomic weight of Scandium is 44.955908 u or g/mol. Melting point of Scandium is 1539 °C and its the boiling point is 2832 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearAbout Scandium
Scandium is a rare metal known also as a transition metal. In its pure form, this metal is quite soft and reactive, especially with water and air. It is a carcinogen and quite a toxic element. Its name is derived from a Latin word meaning Scandinavia. Scandium is rather used for research since it is very rare and can be found only is a few rare types of minerals. As a metal, scandium has very low density, so it is used in alloys with other light metals like aluminum in aircraft industry, etc. Combined with mercury and iodine, scandium is used for producing bulbs.
Properties of Scandium Element

| Atomic Number (Z) | 21 |
|---|---|
| Atomic Symbol | Sc |
| Group | 3 |
| Period | 4 |
| Atomic Weight | 44.955908 u |
| Density | 2.989 g/cm3 |
| Melting Point (K) | 1814 K |
| Melting Point (℃) | 1539 °C |
| Boiling Point (K) | 3109 K |
| Boiling Point (℃) | 2832 °C |
| Heat Capacity | 0.568 J/g · K |
| Abundance | 22 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Transition metal |
| Electronegativity (Pauling) χ | 1.36 |
| Ionization Energy (eV) | 6.5615 |
| Atomic Radius | 160pm |
| Covalent Radius | 144pm |
| Valence Electrons | 2 |
| Year of Discovery | 1879 |
| Discoverer | Nilson |
What is the Boiling Point of Scandium?

Scandium boiling point is 2832 °C. Boiling point of Scandium in Kelvin is 3109 K.
What is the Melting Point of Scandium?
Atomic Number For Scandium To Zinc
Scandium melting point is 1539 °C. Melting point of Scandium in Kelvin is 1814 K.
How Abundant is Scandium?
Abundant value of Scandium is 22 mg/kg.
What is the State of Scandium at Standard Temperature and Pressure (STP)?
State of Scandium is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Scandium Discovered?
Scandium was discovered in 1879.
| Scandium is a silver-white hard metal which develops a slightly yellowish or pinkish cast upon exposure to air. |
Scandium
| Atomic Number: | 21 | Atomic Radius: | 211 pm (Van der Waals) |
| Atomic Symbol: | Sc | Melting Point: | 1541 °C |
| Atomic Weight: | 44.96 | Boiling Point: | 2836 °C |
| Electron Configuration: | [Ar]4s23d1 | Oxidation States: | 3, 2,[2] 1[3] (an amphoteric oxide) |
History
From the Latin word Scandia, Scandinavia. On the basis of the Periodic System, Mendeleev predicted the existence of ekaboron, which would have an atomic weight between 40 of calcium and 48 of titanium.The element was discovered by Nilson in 1878 in the minerals euxenite and gadolinite, which had not yet been found anywhere except in Scandinavia. By processing 10 kg of euxenite and other residues of rare-earth minerals, Nilson was able to prepare about 2g of highly pure scandium oxide. Later scientists pointed out that Nilson's scandium was identical with Mendeleev's ekaboron.
Sources
Scandium is apparently much more abundant (the 23rd most) in the sun and certain stars than on earth (the 50th most abundant). It is widely distributed on earth, occurring in very minute quantities in over 800 mineral species. The blue color of beryl (aquamarine variety) is said to be due to scandium. It occurs as a principal component in the rare mineral thortveitite, found in Scandinavia and Malagasy. It is also found in the residues remaining after the extraction of tungsten from Zinnwald wolframite, and in wiikite and bazzite.
Most scandium is presently being recovered from thortveitite or is extracted as a by-product from uranium mill tailings. Metallic scandium was first prepared in 1937 by Fischer, Brunger, and Grienelaus who electrolyzed a eutectic melt of potassium, lithium, and scandium chlorides at 700 to 800°C. Tungsten wire and a pool of molten zinc served as the electrodes in a graphite crucible. Pure scandium is now produced by reducing scandium fluoride with calcium metal.
The production of the first pound of 99% pure scandium metal was announced in 1960.
Properties
Scandium is a silver-white metal which develops a slightly yellowish or pinkish cast upon exposure to air. A relatively soft element, scandium resembles yttrium and the rare-earth metals more than it resembles aluminum or titanium.
It is a very light metal and has a much higher melting point than aluminum, making it of interest to designers of spacecraft. Scandium is not attacked by a 1:1 mixture of HNO3 and 48% HF.

Sc Element
Chemically it is one of the alkaline earth elements; it readily forms a white coating of nitride in air, reacts with water, burns with a yellow-red flame.
Uses
About 20 kg of scandium (as Sc2O3) are used yearly in the U.S. to produce high-intensity lights. The radioactive isotope 46Sc is used as a tracing agent in refinery crackers for crude oil, etc.
Scandium iodide added to mercury vapor lamps produces a highly efficient light source resembling sunlight, which is important for indoor or night-time color TV.
How Many Electrons Does Scandium Have
Handling
Melting Point Of Scandium
Little is yet known about the toxicity of scandium; therefore it should be handled with care.
