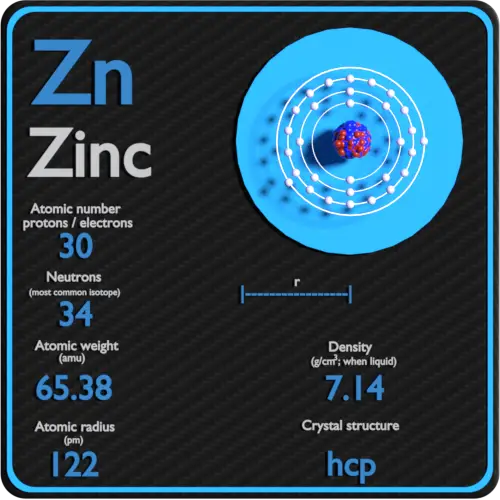
Zinc Mass Number
››More information on molar mass and molecular weight. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Mass Number of Zinc Mass numbers of typical isotopes of Zinc are 64; 66-68; 70. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The mass number for zinc is 65.38amu. The mass number is the mass of one atom and is approximately equal to the number of neutrons added to the. Radioactive Material Identification Common Names: Zinc-65 Atomic Number: 30 Chemical Form: Soluble Chemical Symbol: Zn-65 or 65Zn Mass Number: 65 (35 neutrons) 2. Radiation Characteristics Physical half-life: 243.8 days Specific Activity ( T Bq/g): 305 Principle. Zinc is the 30th element on the periodic table, and has an atomic number of 30. Zinc has a mass number of 65.38. It contains 30 protons and 30 electrons.
Molar mass of Zn = 65.38 g/mol
Convert grams Zn to moles or moles Zn to grams
| Symbol | # of Atoms | Zinc | Zn | 65.38 | 1 | 100.000% |
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Overview
Zinc is a transition metal that occurs in the center of the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. The space between Groups 2 and 13 is occupied by the transition metals. These metals share many physical and chemical properties in common.
Alloys and compounds of zinc have been known since at least 500 B.C. But zinc metal was not known or used until much later. The reason is that zinc boils away or vaporizes easily when heated. Any effort to release zinc from its compounds also causes the metal to evaporate into the air.
Zinc was probably known in Asia before it was discovered in Europe. Ancient books from both India and China refer to zinc products. Such products were imported to Europe from Asia before they were made in Europe.
SYMBOL
Zn
ATOMIC NUMBER
30
ATOMIC MASS
65.38
FAMILY
Group 12 (IIB)
Transition metal
PRONUNCIATION
ZINK
The most important use of zinc today is in galvanizing other metals. Galvanizing is the process of laying down a thin layer of zinc on the surface of a second metal. Zinc does not corrode (rust) as easily as iron and other metals. So the thin layer of zinc protects iron and other metals from corrosion.
Discovery and naming
Some metals can be obtained from their ores easily. In a few cases, all that is needed is to heat the ore. Heating an ore of zinc releases the free metal. But with zinc, there is an additional problem. Zinc metal sublimates very easily. Sublimation is the process by which a solid changes directly to a gas when heated, without first changing to a liquid. Anyone who wanted to make zinc from its ore would lose the zinc almost immediately by sublimation.
Of course, early people did not understand this process. They may very well have made zinc by heating its ores. But any zinc they made would have floated away immediately. Still, a process for extracting zinc from its ores was apparently invented in India by the 13th century. The process involves heating the zinc ore in a closed container. When zinc vapor forms, it condenses inside the container. It can then be scraped off and used. That method seems to have been passed to China and then, later, to Europe.
In the meantime, ancient people were familiar with compounds and alloys of zinc. For example, there are brass objects from Palestine dating to 1300 B.C. Brass is an alloy of copper and zinc. The alloy may have been made by humans or found naturally in the earth. No one knows the origin of the brass in these objects.
The first European to describe zinc was probably Swiss physician Paracelsus (1493-1541). Paracelsus' real name was Theophrastus Bombastus von Hohenheim. Early in life, he took the name Paracelsus, meaning 'greater than Celsus.' Celsus was one of the great Roman physicians. Paracelsus wanted the world to know that he was even 'greater than Celsus.'
Paracelsus was also an alchemist. Alchemy was a kind of pre-science that existed from about 500 B.C. to near the end of the 16th century. People who studied alchemy—alchemists—wanted to find a way to change lead, iron, and other metals into gold. They were also looking for the 'secret to eternal life.' Alchemy contained too much magic and mysticism to be a real science. But it developed a number of techniques and produced many new materials that were later found to be useful in modem chemistry.
Paracelsus first wrote about zinc in the early 1500s. He described some properties of the metal. But he said he did not know what the metal was made of. Because of his report on the metal, Paracelsus is sometimes called the discoverer of zinc.
The name zinc was first used in 1651. It comes from the German name for the element, Zink. What meaning that word originally had is not known.
Physical properties
Zinc is a bluish-white metal with a shiny surface. It is neither ductile nor malleable at room temperature. Ductile means capable of being drawn into thin wires. Malleable means capable of being hammered into thin sheets. At temperatures above 100°C (212°F), however, zinc becomes somewhat malleable.
Zinc's melting point is 419.5°C (787.1°F) and its boiling point is 908°C (1,670°F). Its density is 7.14 grams per cubic centimeter. Zinc is a fairly soft metal. Its hardness is 2.5 on the Mohs scale. The Mohs scale is a way of expressing the hardness of a material. It runs from 0 (for talc) to 10 (for diamond).
Chemical properties
Zinc is a fairly active element. It dissolves in both acids and alkalis. An alkali is a chemical with properties opposite those of an acid. Sodium hydroxide ('common lye') and limewater are examples of alkalis. Zinc does not react with oxygen in dry air. In moist air, however, it reacts to form zinc carbonate. The zinc carbonate forms a thin white crust on the surface which prevents further reaction. Zinc burns in air with a bluish flame.
Occurrence in nature
The abundance of zinc in the Earth's crust is estimated to be about 0.02 percent. That places the element about number 24 on the list of the elements in terms of their abundance.
A process for extracting zinc from its ores was apparently invented in India by the 13th century.
Zinc never occurs as a free element in the earth. Some of its most important ores are smithsonite, or zinc spar or zinc carbonate (ZnCO 3 ); sphalerite, or zinc blende or zinc sulfide (ZnS); zincite, or zinc oxide (ZnO); willemite, or zinc silicate (ZnSiO 3 ); and franklinite [(Zn,Mn,Fe)O (Fe,Mn 2 )O 3 ].
The largest producer of zinc ore in the world today is Canada. Other important producing nations are Australia, China, Peru, the United States, and Mexico. In the United States, more than half of the zinc produced comes from Alaska. Other important producing states are Tennessee, Missouri, Montana, and New York.
Isotopes
Five naturally occurring isotopes of zinc are known. They are zinc-64, zinc-66, zinc-67, zinc-68, and zinc-70. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About eight radioactive isotopes of zinc are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
One radioactive isotope of zinc, zinc-65, has some practical importance. Zinc-65 is used as a tracer to study physical and biological events. A tracer is an isotope whose presence in a system can easily be detected. The isotope is injected into the system at some point. Inside the system, the isotope gives off radiation. That radiation can be followed by means of detectors placed around the system.
For example, zinc-65 is used to study how alloys wear out. An alloy can be made using zinc metal. But the zinc used is zinc-65 instead of ordinary zinc. Changes in radiation given off by the radioactive isotope can be followed to find patterns in the way the alloy wears out. Zinc-65 can also be used to study the role of zinc in the human body. A person can be fed food that contains a small amount of zinc-65. The movement of the isotope through the body can be followed with a detector. A researcher can see where the isotope goes and what roles it plays in the body.
Extraction
As with many metals, pure zinc can be prepared from an ore by one of two methods. First, the ore can be roasted (heated in air). Roasting converts the ore to a compound of zinc and oxygen, zinc oxide (ZnO). The compound can then be heated with charcoal (pure carbon ). The carbon takes the oxygen away from the zinc, leaving the pure metal behind:
The other method is to pass an electric current through a compound of zinc. The electric current causes the compound to break apart. Pure zinc metal is produced.
Uses
The annual cost of corrosion (rusting) in the United States is estimated to be about $300 billion. This is money lost when metals become corroded and break apart. Buildings and
The second largest use of zinc is in making alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. Two of the most common alloys of zinc are brass and bronze. Brass is an alloy of zinc and copper. Bronze is an alloy of copper and tin that may also contain a small amount of zinc. Alloys of zinc are used in a great variety of products, including automobile parts, roofing, gutters, batteries, organ pipes, electrical fuses, type metal, household utensils, and building materials.
Compounds
A number of zinc compounds have important uses. Some examples are the following:
Zinc deficiency can interfere with a plant's ability to reproduce.
zinc acetate (Zn(C 2 H 3 O 2 ) 2 ): wood preservative; dye for textiles; additive for animal feed; glazing for ceramics
zinc arsenate (Zn 3 (AsO 4 ) 2 ): wood preservative; insecticide
zinc borate (ZnB 4 O 7 ): fireproofing of textiles; prevents the growth of fungus and mildew
zinc chloride (ZnCl 2 ): solder (for welding metals); fireproofing; food preservative; additive in antiseptics and deodorants; treatment of textiles; adhesives; dental cement; petroleum refining; and embalming and taxidermy products
zinc fluorosilicate (ZnSiF 6 ): mothproofing agent; hardener for concrete
zinc hydrosulfite (ZnS 2 O 4 ): bleaching agent for textiles, straw, vegetable oils, and other products;
zinc oxide (ZnO): used in rubber production; white pigment in paint; prevents growth of molds on paints; manufacturer of glass; photocopy machines; production of many kinds of glass, ceramics, tile, and plastics
zinc phosphide (Zn 3 P 2 ): rodenticide (rat killer)
zinc sulfate (ZnSO 4 ): manufacture of rayon; supplement in animal feeds; dyeing of textiles; and wood preservative

Health effects
Zinc is an essential micronutrient for plants, humans, and animals. Zinc deficiency has relatively little effect on the health of a plant, but it interferes with reproduction. Pea plants deprived of zinc, for example, will form flowers. But the flowers will not turn to seeds.
In humans, zinc deficiencies are more serious. Zinc is used to build molecules of DNA. DNA is the chemical in our body that tells cells what chemicals they should make. It directs the reproduction of humans also. Fetuses (babies that have not yet been born) deprived of zinc may grow up to have mental or physical problems. Young children who do not get enough zinc in their diet may experience loss of hair and skin lesions. They may also experience retarded growth called dwarfism. Chemists have now found that zinc plays an essential role in the manufacture of many important chemicals in the human body.
Zinc is an essential micronutrient for humans. But too much or too little can cause health problems.
Mass Number Of Zinc 65
On the other hand, an excess of zinc can cause health problems, too. Breathing zinc dust may cause dryness in the throat, coughing, general weakness and aching, chills, fever, nausea, and vomiting. One sign of zinc poisoning is a sweet taste in the mouth that cannot be associated with eating sweet foods. Certain compounds of zinc can be harmful to health also. Zinc chloride (ZnCl 2 ), for example, can cause skin rashes and sore throat.
