Halogen Atom
The halogen atoms possess the highest electron affinities3 3.0–3.6 eV among all the elements. It has been demon-strated that molecules consisting of a metal atom at the core surrounded by halogen atoms possess electron affinities that can be higher than those of the halogen atoms.4–6 This was. Halogen - Halogen - Relative reactivity: The great reactivity of fluorine largely stems from the relatively low dissociation energy, a standard measure for bond energies, of the F―F bond (37.7 kilocalories per mole) and its ability to form stable strong bonds with essentially all the other elements. Fluorine (F2) and chlorine (Cl2) are gases at room temperature. Bromine (Br2) is a reddish. Halogen atoms are more electronegative than carbon, therefore, carbon-halogen bond of alkyl halide is polarised; the carbon atom bears a partial positive charge whereas the halogen atom bears a partial negative charge. As we go down the group in the periodic table, the size of halogen atom increases. Fluorine atom is the smallest and iodine atom is the.
Click to see full answer.

 Subsequently, one may also ask, what are the characteristics of halogens?
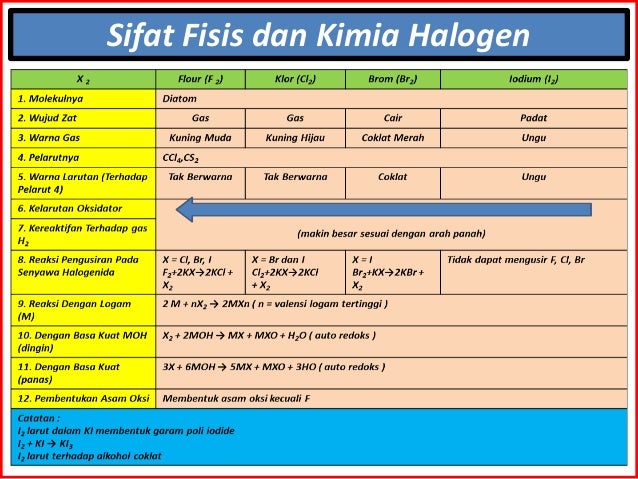
Subsequently, one may also ask, what are the characteristics of halogens?Halogens display physical and chemical properties typical of nonmetals. They have relatively low melting and boiling points that increase steadily down the group. Near room temperature, the halogens span all of the physical states: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid.
Also, what are the three characteristics of halogens? They exist in all three classical states of matter – solid, liquid and gas. All halogens are electronegative. They gain electrons very fast making them most reactive of all chemical elements. Halogens are diatomic when kept under room temperature.
Also, what is a halogen atom?
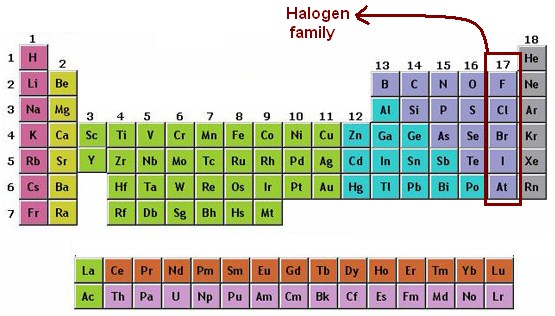
Halogen, any of the six nonmetallic elements that constitute Group 17 (Group VIIa) of the periodic table. The halogen elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts).
What atom has the same characteristics as halogen?
electronegativity: The tendency of an atom to attract electrons to itself. halogens: Group 17 (or VII) in the periodic table consisting of fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They share similar chemical properties.
The organic compounds containing halogen atoms as part of their molecules are referred to as organohalogen compounds. Haloalkanes (alkyl halides), haloarenes (aryl halides), acid halides are some important class of these compounds.
Their molecules contain polar C-X bond as functional group; where X is a halogen atom like F, Cl, Br and I. The carbon atom carries partial positive charge and halogen atom, being more electronegative than carbon, carries partial negative charge.
The organohalogen compounds find many applications in day to day life and in industry. They are used as solvents in laboratory and in industries (like chloroform, carbon tetrachloride, westron, westrosol); as precursors to other organic molecules; as anesthetics (e.g. halothane); as refrigerants (like freons); etc.
Chloramphenicol, an antibiotic containing halogen is used for the treatment of typhoid fever. Thyroxine is an iodine containing harmone produced by thyroid glands. Polyvinylchloride, PVC is a polymer containing chlorine atoms.
Simple organohalogens like alkyl halides are obtained by replacing one or more H atoms by X atom(s) from hydrocarbons. These compounds can be classified based on hybridization of carbon to which the halogen is bonded.
Halogen Atomic Radius
Classification based on hybridization of carbon
Compounds containing sp3 C–X Bond
This class of organohalogen compounds includes:
i) Alkyl halides (or haloalkanes)
ii) Allylic halides
iii) Benzylic halides
Alkyl halides (or haloalkanes)
Haloalkanes are represented by R–X. The X atom is bonded to sp3 hybridized carbon of an alkyl group, R that is derived from an alkane.
Monohaloalkanes are represented by general formula CnH2n+1X. These are obtained by replacing one of the hydrogen from alkane by halogen.
| Alkane | minus H plus X = | Mono haloalkane |
| CnH2n+2 | - H + X = | CnH2n+1X |
For example, methyl chloride, CH3-Cl is obtained by replacing one hydrogen atom of methane by chlorine.
CH4 - H + Cl = CH3-Cl
They are further classified into primary (1o), secondary (2o) and tertiary halides (3o) according to the nature of carbon to which halogen is bonded.
E.g.
Primary: ethyl chloride - the chlorine atom is attached to primary carbon.
Secondary: isopropyl chloride - the chloro group is attached to a secondary carbon.
tertiary: tert-butyl chloride - the chlorine atom is bonded to a tertiary carbon.
Allylic halides
In allylic halides, the halogen is bonded to an sp3–hybridized carbon atom that is adjacent to a C=C. This carbon is also know as allylic carbon.
E.g. Allyl chloride.
They show special trend in the reactivity due to stability of allyl carbocation.
Benzylic halides
In benzylic halides, the halogen group is bonded to an sp3-hybridized benzylic carbon atom that is inturn bonded to an aromatic ring.
E.g. Benzyl bromide.
These are also more reactive than normal haloalkanes due to stability of benzyl carbocations.
Compounds containing sp2 C–X bond
This class includes :
Halogen Atomic Mass
i) Vinylic halides
ii) Aryl halides
iii) Acid halides
Vinylic halides
The halogen atom is bonded directly to the sp2-hybridized carbon of a C=C.
E.g. vinyl fluoride.
Aryl halides (Haloarenes)
In Haloarenes, the halogen atom is bonded directly to the sp2-hybridised carbon atom of an aromatic ring. These are also referred to as haloarenes.
E.g. Chlorobenzene
Both vinylic and aryl halides are less reactive towards nucleophilic substitution reactions due to stronger C-X bond.
Acid halides
Acid halides are the derivatives of carboxylic acids. These are obtained by substituting -OH group of carboxylic acid by any halogen atom. In this case, the X atom is attached to an sp2 hybridized carbonyl carbon.
The chemistry of these compounds is discussed along with other acid derivatives separately.
Polyhalogenated compounds
The organohalogen molecules may contain one or more halogens also. They are referred to as dihalo, trihalo, tetrahalo, poly halo compounds.
Dihalo substituted compounds:
Avm network & wireless cards driver. E.g. Methylene chloride, CH2Cl2
If two halogen groups are present on one carbon, those are termed as geminal dihalides (e.g. Ethylidene dichloride) and if two halogen atoms are present on adjacent carbon atoms, then those are referred to as vicinal dihalides (e.g. Ethylene dichloride).

Trihalo substituted:
E.g.
Chloroform, CHCl3,
Westrosol (or Trichloro ethylene), CHCl=CCl2
Tetrahalo substituted:
E.g.
Carbontetrachloride, CCl4
Westron (or Acetylene tetrachloride), CHCl2-CHCl2
Halogen Atomic
Perhalo compounds: When all the H atoms are substituted by X atoms, those compounds are generally referred to as perhalo compounds.
Halogen Atoms
E.g. Perchlorodiethyl ether
| Organic chemistry: Home page | Nomenclature of organohalogen compounds > |
